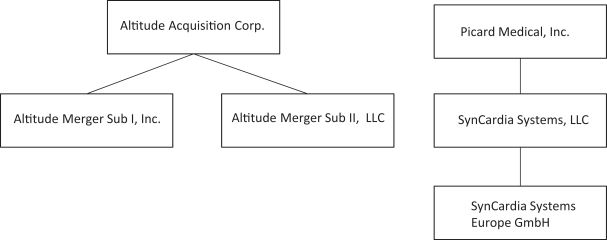
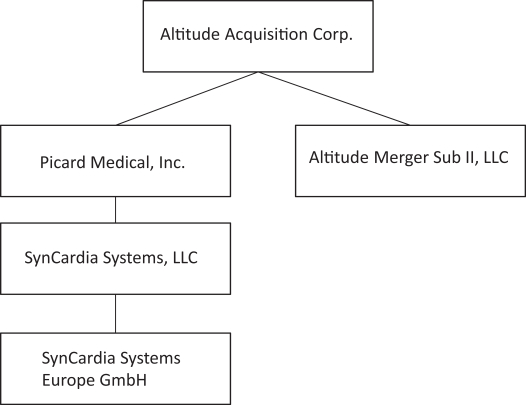
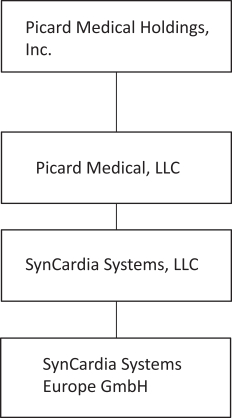
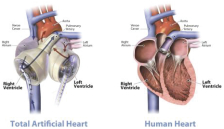
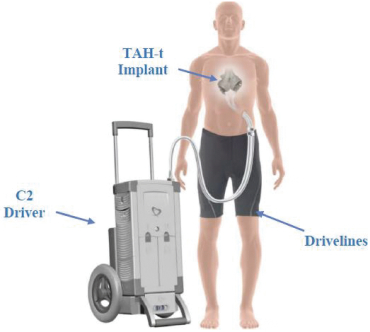
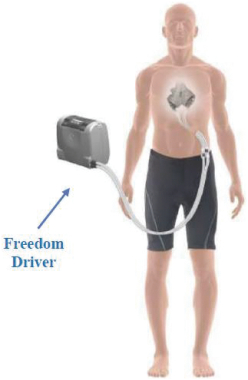
On April 7, 2023, Altitude’s stockholders approved a further amendment to the
Current Charter to extend the date by which Altitude must complete a business combination from April 11, 2023 monthly up to eight (8) times for an additional one month each time, up to December 11, 2023. In connection with the
amendment, Altitude offered public stockholders right to have their public shares converted into a pro rata portion of the Trust Account and stockholders holding an aggregate of 337,457 public shares exercised their right to redeem their shares for
approximately $10.08 per share, or a total of approximately $3.4 million of the funds held in Altitude’s Trust Account.
Results of Operations
As of June 30, 2023, we have not commenced any operations. All activity for the period from August 12, 2020 (inception) through
June 30, 2023, relates to our formation and IPO, and, since the completion of the IPO, search for a target to consummate a business combination, including the negotiation of the Business Combination Agreement with Picard. We will not generate any
operating revenues until after the completion of a business combination, at the earliest. We generate non-operating income in the form of interest income on cash deposits in the Trust Account. We incur expenses as a result of being a public company
(for legal, financial reporting, accounting and auditing compliance), as well as for due diligence expenses.
For the three months ended
June 30, 2023, we had a net loss of $1,658,466 which included general and administrative costs of $1,799,105, unrealized loss on change in fair value of warrants of $98,324 and provision for income taxes $38,301, offset by interest income earned on
the operating bank account of $18 and interest income earned on the proceeds in the Trust Account of $277,246.
For the six months ended
June 30, 2023, we had a net loss of $3,107,201 which included general and administrative costs of $3,418,295 and provision for income taxes $38,301, partially offset by unrealized gain on change in fair value of warrants of $72,111, interest income
earned on the proceeds in the Trust Account of $277,246 and interest income earned on the operating bank account of $38.
For the three
months ended June 30, 2022, we had a net loss of $234,912 which included general and administrative costs of $1,518,182 and income tax provision of $8,780, partially offset by unrealized gain on change in fair value of warrants of $987,781 and
interest income earned on the proceeds in the Trust Account of $304,269.
For the six months ended June 30, 2022, we had a net income of
$8,382,279 which included unrealized gain on change in fair value of warrants of $10,525,589, interest income earned on the proceeds in the Trust Account of $311,869 and interest income earned on the operating bank account of $1, partially offset by
general and administrative costs of $2,446,400 and income tax provision of $8,780.
For the year ended December 31, 2022, we had a net
income of $9,342,644 which included unrealized gain on change in fair value of warrants of $12,065,834, interest income earned on the proceeds in the trust account of $654,735 and interest income earned on the operating bank account of $4, partially
offset by general and administrative costs of $3,339,747 and income tax provision of $38,180.
For the year ended December 31, 2021, we
had a net income of $15,225,829, which included unrealized gain on change in fair value of warrants of $20,358,180, gain on settlement of payable of $860,699, interest income earned on the proceeds in the trust account of $26,714 and interest income
earned on the operating bank account of $24, partially offset by general and administrative costs of $6,019,788.
For the year ended
December 31, 2021, the Company recorded a gain on settlement for a payable of $860,699. This gain was generated by a forgiveness of legal costs associated with the year ended December 31, 2021 being reduced from $4,594,437 to $3,733,738.
185